13.05.2016
Certificate of Origin Policy (TSE/BSE)
What is the purpose of this Policy and FAQ?
To provide information and guidance on issues related to the origination-traceability of Sigma-Aldrich
products, particularly those from animal source.
Policy Statement
Sigma-Aldrich recognizes that product traceability is an important requirement for our customers. To
meet this requirement, we actively engage our suppliers to provide source and processing information for raw materials used in manufacturing and for materials purchased for resale. That information is compiled into Certifi cate of Origin documents, which can be accessed via our Web site at sigma-aldrich.com, Technical Support, or by contacting your local representative.
A Sigma-Aldrich Certifi cate of Origin provides batch origin or source information about a product so customers can perform a TSE/BSE risk assessment based upon its intended use in their specifi c application.
Product-specifi c validated removal studies have not been conducted for the majority of catalog-offered items. Statements to confi rm that a product is “free of TSE/BSE” are not scientifi cally possible; consequently, Sigma-Aldrich cannot guarantee that our products are free of TSE or BSE materials. However, we will provide data that allow our customers to evaluate and minimize risk when using our products in their production processes.
If Sigma-Aldrich holds a Certifi cate of Suitability (CEP) for a manufactured product, the CEP registration number will be reported on the Certifi cate of Origin. If a supplier of Sigma-Aldrich holds the Certificate of Suitability for a purchased product, the information can be located at www.edqm.org for the appropriate third-party CEP registration number. The supplier’s number will not appear on the Sigma-Aldrich Certifi cate of Origin document.
To accommodate our customers’ needs, we will make every reasonable effort to obtain Certifi cate of
Origin information. We encourage customers with TSE compliance requirements to contact us within
1 year of purchase to increase the opportunity for obtaining the needed information.
Sigma-Aldrich Quality and Compliance Management, August 3rd, 2005.
Frequently Asked Questions (FAQ)
Why is TSE/BSE a concern?
The neurodegenerative diseases are caused by a Prion (PrPsc). This Prion (PrPsc) is an infectious protein without DNA or RNA. The host precursor of this infectious protein is a non-infectious agent, which is present in all animal species (including man). While transmission mechanisms of this Prion (within a single species or from one species to another species) are not well known, the transmission minimization of those Prions through pharmaceutical products is a major concern.
Prion (PrPsc) Background Information
• Prions are chemicals, like endotoxin
• Prions are not living organisms
• Presence of Prions is not easily demonstrated with a diagnostic test (current available testing is very
limited, only done at certain laboratories on the actual tissue not on extracted fi nal products, e.g.,
enzyme)
• Prions adhere very tenaciously to surfaces—making them hard to remove; they can be re-deposited
on other surfaces during processing.
• Prions are resistant to protease treatment, certain chemical agents, and heat denaturation.
What can be done to minimize this risk?
Risk Assessment is considered an acceptable means to demonstrate that the presence of PrPsc is minimized. Per EMEA/410/01, “…the measures taken to manage the risk of transmitting animal TSEs via medicinal products represent risk minimization rather than risk elimination. Consequently, the basis for regulatory compliance should be based on a risk assessment, taking into consideration all pertinent factors…”
• Sourcing
- Sourcing animals from countries considered to be least risk (i.e., GBR I)
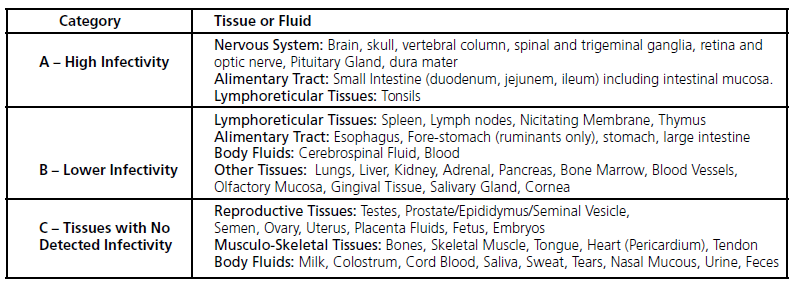
- Certain tissues have a higher risk (i.e., brain, spinal cord) vs. lower risk (i.e., milk, wool)
• Manufacturing process
- Exclude presence of any animal or human-derived material in production process (raw materials,
reagents, contamination of equipment)
- Dedicated line/equipment, slaughtering without brain penetration
What is Sigma-Aldrich doing to support this Risk Management approach?
1. Collecting Traceability/Processing Information
For Sigma-Aldrich catalog-listed products, we are collecting information from our suppliers and internal
manufacturing sites to provide verifi ed source and manufacturing information. This collected informationis presented in a Sigma-Aldrich Certifi cate of Origin document by batch. This information can include,but is not limited to, the following:
• Is product Synthetic, Biological (i.e., animal, plant, human), or from a non-living Natural source?
• Were only Synthetic materials used during the manufacturing/packaging process?
• If product is Biologic or if Biologic-sourced material was used in the manufacturing process;
- Species (i.e., Bovine, Porcine)
- Tissue (i.e, Brain, Lung, Blood)
- Country where the animal originated and tissue was collected
- Feeding and Slaughter method
• Means for controlling cross contamination (i.e., dedicated equipment, recognized cleaning/sanitization
process)
• Quality Management Systems (i.e., ISO, cGMP)
2. Manufacturing Process
For internal manufactured items derived from animal source or where animal source is used in processing, site-specifi c activities are being employed to minimize risk and would be reported on the Certifi cate of Origin for that specifi c product/batch. These can include, but are not limited to, the following:
• Where feasible, sourcing tissues from low-risk GBR I countries (i.e., New Zealand)
• Where feasible, using manufacturing process absent of human/animal-sourced material
• Where possible, dedicated equipment or segregated areas/lines
• For non-dedicated equipment, employing cleaning/sanitization procedures to minimize cross
contamination
- 1 N NaOH for ;1 hr exposure (per WHO guidelines)
- ;1.6% CIP-100 (Steris Corp.) caustic detergent for ;43 °C for ;15-minute exposure period
Fichet, G. et al., Novel methods for disinfection of prion-contaminated medical devices.
Lancet, vol. 364 (9433) p. 521–526. (2004).
3. For a few selected products, Sigma-Aldrich is the holder of Certifi cates of Suitability for TSE risk.
Purpose of Certifi cates of Suitability (CEP)
CEP’s are recognized by 34 signatory states of the European Pharmacopoeia Convention and by the
European Union. Other countries have also chosen to recognize them. CEP can be used by the manufacturers of pharmaceutical products in their applications for marketing authorization to demonstrate the compliance of the substance used with the monographs of the European Pharmacopoeia and Directives 2001/83/EC and 2001/82/EC.
What does the procedure include?
The EDQM must be sent a full dossier describing in detail the manufacturing method of the substance
and the impurities that are associated with it, and/or the countries of origin, the type of animal tissues
and the quality assurance, so that the reference to the European Pharmacopoeia can be validated. Thedossier is processed according to a procedure that guarantees its confi dentiality and it is assessed by independent experts whose impartiality is guaranteed by their status and a confi dentiality agreement.
Who is the procedure for?
Manufacturers, whatever their location in the world, (or the duly authorized representatives of these
manufacturers) of substances, obtained by synthesis, extraction or fermentation, and substances
concerned by TSE risk. Suppliers of any substances with TSE risk used in the production or preparation of medicinal products can apply for a certifi cate concerning the evaluation of the reduction of TSE risk according to the general monograph. This certifi cate can then be used by manufacturers of medicinal products in their marketing authorizations for demonstration of compliance with Directives 2001/83/ECand 2001/82/EC.
What current products does Sigma-Aldrich have registered with the EDQM?
• 31 CEP’s for 46 products, i.e., enzymes, serum (Ref: www.edqm.org)
• Sigma-Aldrich and SAFC-JRH Biosciences brands
• Contact your Sigma-Aldrich representative for details
When does Sigma-Aldrich submit a new CEP dossier to the EDQM?
• Most catalog-offered products do not warrant consideration for submission, however
• Where a product has the potential higher TSE risk due to source i.e. Bovine-Category A tissue,
Sigma-Aldrich will consider pursuing a CEP submission with the EDQM in conjunction with the
requesting customer’s business and/or regulatory requirements. Contact your Sigma-Aldrich
representative for details.
Why does Sigma-Aldrich not register more products with the EDQM?
• Majority of offered products are intended for research use only
• Expensive: requires fees for initial submission, each revision, and renewal
• Dossier includes: specifi cations, written analytical methods, defi ned manufacturing process, impurities, inactivation steps, cleaning/sanitization process, any validation, dedicated equipment, etc.
• Very long submission process: average 20 months and requires continuous monitoring for any
changes (raw material, site, production process)
• If monograph revised, affected product will need to be reviewed to determine compliance with
new requirements
For products that are marketed as Animal Component-Free, how is that defined?
Animal source materials are primarily a concern with regard to Transmissible Spongiform Encephalopathy (TSE) and viral contamination. Products manufactured by Sigma-Aldrich and classifi ed as animal component-free will not contain or use in the manufacturing process, any primary raw materials derived directly from bovine or other animal tissues1. This applies to all aspects of product manufacturing.
Secondary raw materials are defi ned as non-animal, but may be derived from processes which include tertiary level materials from animal components classifi ed as very low risk (Category IV as defi ned by the European Medicines Agency2 or Category C as defi ned by the World Health Organization). Based on the positions of the European Medicines Agency and the World Health Organization, Sigma-Aldrich extends the defi nition of animal component-free to include tertiary materials, which are of “no detected infectivity” i.e., Category C tissues. We conclude that since none of the raw materials used in the manufacture are derived directly from bovine or any other animal tissues, and any secondary or tertiary level raw material will be sourcedfrom either synthetic or Category IV/C or non-TSE relevant animal species (e.g., pigs and birds), Sigma-Aldrich products classifi ed as animal component-free pose a negligible risk of transmitting TSE agents.

Source: Certifi cate of Origin Policy (TSE/BSE) 01-000-014 Rev. 1Effective Date: August 3rd, 2005