01.12.2015
Certified Reference Materials from Cerilliant and Certificate of Analysis Examples
For over 30 years, Cerilliant has been dedicated to providing Certified Reference Materials (CRMs) for various testing applications, offering over 3000 catalog reference standards as well as OEM and custom products and services. Cerilliant sustains a modern, robust quality system which incorporates cGMP, GLP, and ISO requirements. Quality credentials include accreditations to ISO Guide 34 and ISO/IEC 17025, certifications to ISO 13485 and ISO 9001, and compliance with ISO 17511 and ISO 15194.
Cerilliant serves a diverse group of clients across many industries, ranging from private and government laboratories, hospitals, research institutes, and analytical instrument and medical device manufacturers to pharmaceutical companies and CRO's — organizations that require reference materials of the highest quality, whether they're conducting forensic or clinical toxicology analysis, diagnostic testing, therapeutic drug monitoring, environmental analysis, pharmaceutical research, or developing new testing devices.
Single-Component Solution Standard

Page 1
1.Expiration Date/Retest Date – Expiration Dates are assigned to established products and are determined through real time stability studies. The Expiration Date defines the total shelf life of the product. Cerilliant discontinues sales of these items 12 months prior to the stated Expiration Date.
2.Solution Purity – Extensive process controls are employed during manufacturing to ensure no degradation or contamination occurs. Analytical verification of solution purity post ampouling provides absolute confirmation.
3.Concentration & Uncertainty – Concentration of the gravimetric preparation expressed as 1.000 ± 0.006 mg/mL Represents the actual concentration (not theoretical) based on material weighings and material Purity Factor
4.Uncertainty Statement – Provides details of what standards were used to develop the uncertainty value, the confidence interval, the coverage factor and the processes or steps incorporated in the uncertainty value. 'Uncertainty of the concentration is expressed as an expanded uncertainty in accordance with ISO 17025 and ISO Guide 34 at the approximate 95% confidence interval using a coverage factor of k = 2 and has been calculated by statistical analysis of our production system and incorporates uncertainty of the purity factor, material density, and mass'.
5.Analytical Verification of Concentration & Ampoule to Ampoule Consistency – The gravimetrically prepared concentration is verified analytically by comparison to an independently prepared calibration solution. Lot-to-lot consistency is demonstrated through analysis of the previous lot (where available). Ampoule-to-ampoule consistency, or lot homogeneity, ensures consistency in recovery and is demonstrated through analysis of ampoules pulled from across the lot. Lack of variability is demonstrated through the tight %RSD reported for the analysis. Acceptance criteria incorporates variability of the analysis.
6.Traceability Statement – Traceability Statement describing traceability to SI units 'Gravimetrically prepared using qualified balances calibrated semi-annually by a high quality balance producer using NIST traceable weights. Calibration verification performed weekly and prior to each use utilizing NIST traceable weights. Each balance has been assigned a minimum weighing by a high quality balance producer taking into consideration the balance and installed environmental conditions to ensure weighing complies with USP tolerances of no more than 0.1% relative error'. Manufacture of Cerilliant standards is fully documented in a detailed batch record capturing all equipment utilized providing traceability to equipment calibration records. Calibration verification weigh tapes are included in each batch record.
Page 2
7.Solution Standard Assay – Shows method used to assay solution to an independently prepared calibration solution and calibration solution qualification data.
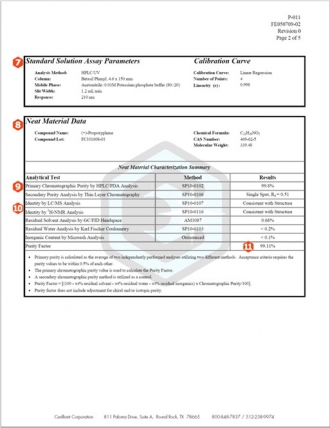
8.Neat Material Characterization Summary and Purity Factor Assignment – Cerilliant Certified Solution Standards begin with full characterization of the neat material including chromatographic purity and analyses for residual content including: water, solvent, and inorganic content. This approach ensures accuracy of the standard and its suitability for demanding quantitative applications. Summary table shows results of all analyses performed and the calculated Purity Factor. Chemical information about the compound and its lot number are also provided for complete traceability.
9.Chromatographic Purity – Cerilliant utilizes multiple techniques to determine chromatographic purity. Results must agree within 0.5% of each other. The Primary purity method is used for purity factor calculations. The Secondary purity method is utilized as a confirming method. This approach ensures proper resolution of impurities and related substances and protects against random analytical error that could result in improper purity assignment.
10.Identity – Cerilliant utilizes multiple methods to determine neat material identity, usually two of the following: NMR, LC/MS, GC/MS, FTIR. Each result must be consistent with compound structure. Spectral data is provided on subsequent pages of the COA. This approach is consistent with ISO Guide 34 requirements and protects against random analytical error that could result in improper identification and ensures complete traceability of the reference standard.
11.Purity Factor – The purity factor (PF) mass balance measurement equation is used to calculate the amount of analyte required to achieve an accurate concentration of the solution standard, accounting for chromatographic purity and residual water, solvent, and inorganic content.
Page 3
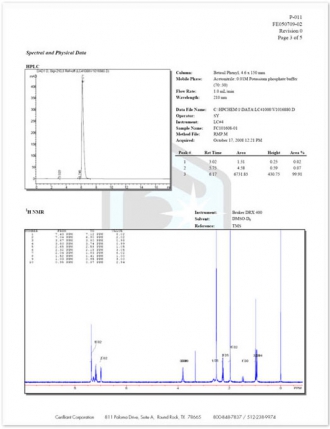
12.Spectral and Physical Data – Details of all neat material testing performed is provided along with run conditions and spectra.

Page 4
13.Spectral and Physical Data (cont.) – Details of all neat material testing performed is provided along with run conditions and spectra.
Page 5
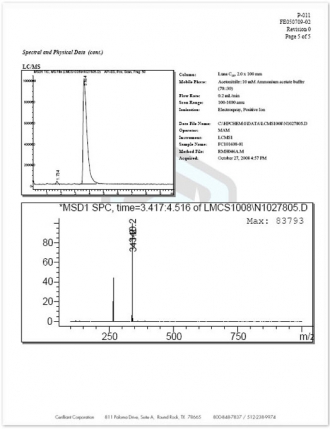
14.Spectral and Physical Data (cont.) – Details of all neat material testing performed is provided along with run conditions and spectra.
Ethanol Solution Standard

Page 1
1.Expiration Date – Expiration Dates are established through real-time stability studies.
2.Solution Purity – Extensive process controls are employed during manufacturing to ensure no degradation or contamination occurs. Analytical verification of solution purity post ampouling provides absolute confirmation.
3.Certified Concentration – The gravimetrically prepared concentration is the 'True Value' and is reported in terms of concentration per volume with stated uncertainty as ±mg/dL. Represents the actual concentration (not theoretical) based on material weighings and material Purity Factor.
4.Uncertainty Statement – Provides details of what standards were used to develop the uncertainty value, the confidence interval, the coverage factor and the processes or steps incorporated in the uncertainty value. Uncertainty of the concentration, expressed in terms of volume, is an expanded uncertainty in accordance with ISO 17025 and ISO Guide 34 at the 95% confidence interval using a coverage factor of k=2 and has been calculated by statistical analysis of our production methods applicable to ethanol reference standards and incorporates uncertainty of the purity factor, material density and mass measurement. The dispensing process is sufficiently controlled as to not be a significant contributor to uncertainty calculations and is, therefore, excluded. Solution stability is established through real time stability studies and is, therefore, excluded. When expressed in percentage terms, the relative standard uncertainty of the concentration is 0.175% and the relative expanded uncertainty is 0.35% at the 95% confidence interval (k=2).
5.Traceability Statement describing traceability to SI units – 'This standard has been prepared and certified under the ISO Guide 34 and ISO/IEC 17025 standards and meets the requirements of a Certified Reference Material as defined by ISO.' The traceability section provides details of each step of the process and how it is traceable to SI units including the certification of the neat ethanol, the gravimetric preparation, dispensing, and certification.

Page 2
6.Solution Standard Analytical Verification – Comparison to NIST SRM – Solution standard concentration is verified analytically using a validated Headspace GC/FID method with known uncertainty by comparison to an appropriate NIST SRM. Acceptance criteria =±2% A calibration control is also used in the analysis. The Control is made from a unique lot of neat ethanol which has been certified. Control has been qualified to NIST SRM. Relative standard uncertainty of the Headspace GC/FID analytical method is 1.675% and includes both uncertainty of the analytical method and uncertainty of the NIST SRM concentration.
7.Ampoule to Ampoule Consistency (lot Homogeneity) – Homogeneity across the lot is verified by testing samples pulled from across the lot. Numerous samples pulled during dispensing utilizing a stratified random sampling plan are tested. %RSD of the analysis across numerous samples represents lot homogeneity. The %RSD of the Prior Lot (shown on the 2nd line) represents system suitability on the date of analysis. Triplicate injections of the Prior Lot are bracketed at the beginning and end of the sequence. %RSD criteria ensures proper system performance throughout the sequence.
8.Neat Material Analysis & Purity Factor – Provides data on characterization of neat ethanol including chromatographic purity and residual water analyses. Residual water analysis is important due to the hygroscopic nature of ethanol. The purity factor (PF) mass balance measurement equation is used to calculate the amount of analyte required to achieve an accurate concentration of the solution standard, accounting for chromatographic purity and residual water.
9.Solution Standard Assay – Shows method used to assay solution to NIST SRM.
Multi-Component Drug Standard

Page 1
1.Retest Date – Retest Dates are assigned to ensure products are continually evaluated during the expected period shelf-life. Expiration Dates are established through real-time stability studies. Refer to Expiration/Retest policy for additional information.
2.Regulatory – States whether controlled or exempt by USDEA and Health Canada - now incorporated on COAs of products containing USDEA controlled substances. All other countries, consult your regulatory authorities.
3.Solution Purity – Extensive process controls are employed during manufacturing to ensure no degradation or contamination occurs. Analytical verification of the purity of each analyte is performed post ampouling to provide absolute confirmation.
4.Concentration & Uncertainty – Concentration of each analyte in the gravimetric preparation expressed as 100.0 ±0.6 µg/mL Represents the actual concentration (not theoretical) based on actual material weighings and the analyte Purity Factor.
5.Uncertainty Statement – Provides details of what standards were used to develop the uncertainty value, the confidence interval, the coverage factor and the processes or steps incorporated in the uncertainty value. 'Uncertainty of the concentration is expressed as an expanded uncertainty in accordance with ISO 17025 and ISO Guide 34 at the approximate 95% confidence interval using a coverage factor of k = 2 and has been calculated by statistical analysis of our production system and incorporates uncertainty of the purity factor, material density, and balance and weighing technique'.
6.Traceability Statement describing traceability to SI units & NIST – Gravimetrically prepared using qualified balances calibrated semi-annually by a high quality balance producer using NIST traceable weights. Calibration verification performed weekly and prior to each use utilizing NIST traceable weights. Each balance has been assigned a minimum weighing by a high quality balance producer taking into consideration the balance and installed environmental conditions to ensure weighing complies with USP tolerances of no more than 0.1% relative error. Concentration is verified against an independently prepared calibration curve gravimetrically prepared using balances calibrated to NIST. Manufacture of Cerilliant standards is fully documented in a detailed batch record capturing all equipment utilized providing traceability to equipment calibration records. Calibration verification weigh tapes are included in each batch record. In addition, each neat material utilized has been identified and thoroughly characterized through the use of multiple analytical techniques. Spectral data is provided on subsequent pages of the COA.

Page 2
7.Solution Standard Assay – Shows method used to assay solution to an independently prepared calibration solution and calibration solution qualification data.
8.Analytical Verification of Concentration & Ampoule to Ampoule Consistency – The gravimetrically prepared concentration is verified analytically through multiple analyses by comparison to an independently prepared calibration solution. Ampoule-to-ampoule consistency, or lot homogeneity, ensures consistency in recovery and is demonstrated through analysis of ampoules pulled from across the lot using a random stratified sampling plan. Lack of variability is demonstrated through the tight %RSD reported for the analysis. Acceptance criteria incorporates variability of the analysis. Lot-to-lot consistency is demonstrated through analysis of the previous lot (where available).
9.Solution Standard Analysis – Spectra of solution standard analysis with peak identification. This solution standard was analyzed by LC/MS.
Page 3
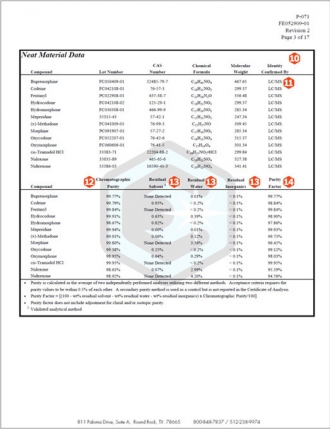
10.Neat Material Characterization Summary and Purity Factor Assignment – Cerilliant Certified Solution Standards begin with full characterization of the neat analytes including chromatographic purity and analysis for residual content including: water, solvent, and inorganic content. This approach ensures accuracy of the standard and its suitability for demanding quantitative applications. The summary table shows results of all analyses performed and the calculated Purity Factor. All testing data, including spectra, is provided on subsequent pages of the COA. In addition we provide the lot number of each neat analyte used ensuring full traceability.
11.Identity – Cerilliant utilizes multiple methods to determine neat material identity, usually two of the following: NMR, LC/MS, GC/MS, FTIR. Each result must be consistent with compound structure. Spectral data is provided on subsequent pages of the COA. This approach is consistent with ISO Guide 34 requirements and protects against random analytical error that could result in improper identification and ensures complete traceability of the reference standard.
12.Chromatographic Purity – Cerilliant utilizes multiple techniques to determine chromatographic purity. Results must agree within 0.5% of each other. The Chromatographic Purity shown in the table is the primary purity method which is used for purity factor calculations. A secondary purity method is utilized as a control but not reported on the multi-component COA. This approach ensures proper resolution of impurities and related substances and protects against random analytical error that could result in improper purity assignment.
13.Residual Content Analysis – Chromatographic purity is only part of the story. Each analyte utilized is fully characterized for non-chromatographic impurities as well. The COA provide full details of results of each analysis for residual solvent, residual water, and residual inorganics for each analyte included in the solution standard. Residual content analysis is critical to calculation of the Purity Factor and to ensuring an accurate quantitative reference standard.
14.Purity Factor – The purity factor (PF) mass balance measurement equation is used to calculate the amount of each analyte required to achieve an accurate concentration of the solution standard, accounting for chromatographic purity and residual water, solvent, and inorganic content.
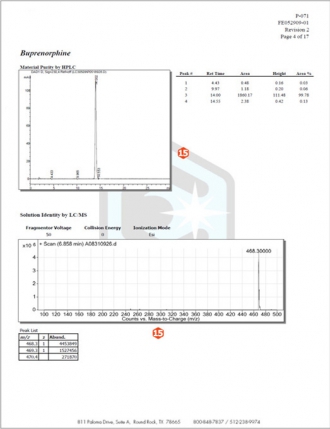
Page 4
15.Neat Material Characterization Details – Subsequent pages of the COA include details of all neat material testing performed on each analyte in the solution along with run conditions and spectra.
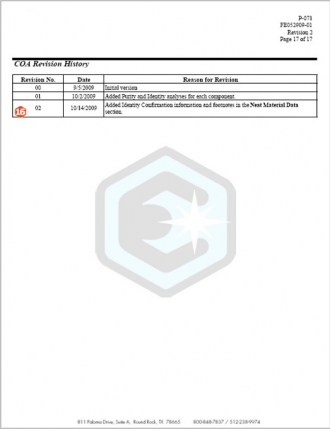
Page 5
16.Revision History – Each new lot begins as Revision 0. When an update to a COA is made for any reason, the revision number will change sequentially. The reason for each revision is included in the revision history table located on the last page of the COA. Reasons for revision may include updates to a Retest or Expiry date, correction of errors, or changes in format. Customers signed up for electronic notification will be notified anytime the COA of a product they purchased within the prior twelve month period is revised.
Source: http://www.sigmaaldrich.com